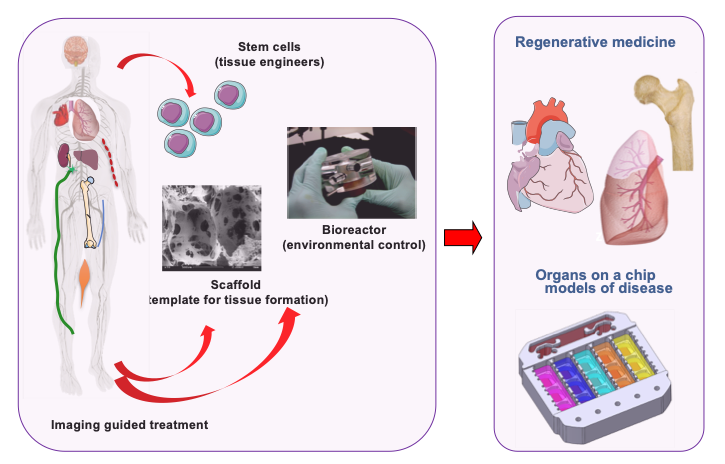
Both in vivo (during development, regeneration, and disease) and in vitro (in engineered tissues), cells interact with the entire context of their environment – the regulatory molecules, other cells, extracellular matrix and physical factors, with synergistic and competing effects. Our laboratory is interested in investigating these interactions at different length and time scales, and utilizing them to engineer functional tissues. To establish controllable and physiologically meaningful conditions, we are developing engineering tools that enable human cells (the “tissue engineers”) to assemble into functional tissues: specialized scaffolds (serving as templates for tissue formation) and bioreactors (providing environmental control, regulatory signals, and on-line insights into cellular/tissue outcomes). We utilize this approach to bioengineer a number of human tissues and organs, for two different applications: (i) regenerative medicine, to repair a tissue/organ lost to injury or disease, and (ii) “organs on a chip” platforms, to study human physiology in health and disease. The engineering designs are driven by the clinical need (to facilitate translation) and are based on biological principles (to provide a realistic biological context for experimental studies). Our diverse research team is collaborating with investigators from many different disciplines, at Columbia, nationally and internationally. Our laboratory is located in the Columbia University Irving Medical Center, and is a state-of-the-art facility for basic and translational studies of cell and tissue engineering. We also host the NIH Tissue Engineering Resource Center and the Center for Dental and Craniofacial Research.
Contact Us
Research Projects
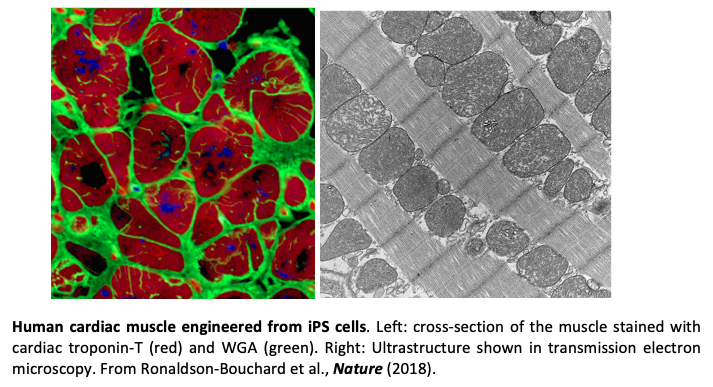
Heart disease remains a major health problem all around the world. Because the current interventions show only limited ability to repair the damaged heart, advances in mechanistic understanding and treatment of cardiac injury would have major clinical significance. Cardiac tissues bioengineered from human iPSCs and matured towards an adult-like phenotype are emerging as highly controllable and increasingly predictable human models for quantitative studies of cardiac injury, disease and regeneration. Our lab has been interested in developing highly advanced bioengineered models of vascularized human cardiac muscle for quantitative patient-specific studies focused on three critical questions: (1) Can we bioengineer vascularized cardiac muscle? (2) Can we develop patient-specific models of cardiac injury-regeneration? (3) Can we identify some of the mechanisms of cardiac injury and regeneration and establish new clinically effective therapeutic regimens? To this end, we engineered cardiac tissues from human iPS cells with adult-like gene expression profiles, remarkably organized ultrastructure, networks of T-tubules, oxidative metabolism, a positive force–frequency relationship, and functional calcium handling (Ronaldson-Bouchard et al., Nature 2018). Such advanced maturation of the tissue was necessary for modeling cardiac physiology and disease. We also showed that the extended delivery of exosomes secreted by iPSC- cardiomyocytes protected the animal hearts from ischemic injury (Liu et al., Nature Biomed Eng, 2018).
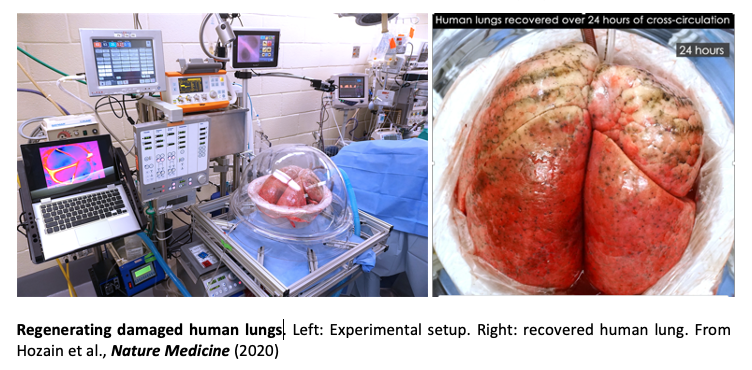
Nearly 25 million people in the U.S. suffer from end-stage lung disease. Lung transplantation, the only curative option for these patients, remains hampered by donor organ shortages, long-term rejection, and the need for immunosuppression. Engineering a human lung remains elusive because of its complexity, with hierarchical vascular and bronchial architectures that create a large surface area for gas exchange and contain many types of cells. We therefore focus on increasing the number of lungs available for transplantation, by bringing the functionality of low-quality donor lungs to the levels required for transplantation. Our approach is to identify and treat the injured regions by removing defective cells while preserving the composition, architecture, and mechanical properties of the extracellular matrix and the surrounding tissue and vasculature. In recent studies we extended the duration of extracorporeal lung support and recovery to as long as 100 hours, using cross-circulation of blood between the living host and the ventilated lung (Hozain et al., J Thor. Cardiovasc Surg., 2020). We showed that this platform enables regeneration of acutely injured human lungs declined for transplantation (Hozain et al., Nature Medicine 2020). Using the tools for lung bioengineering and animal models, we are also developing models of pulmonary fibrosis, that allow us controlled studies of the roles of cells and extracellular matrix in the initiation and progression of disease.
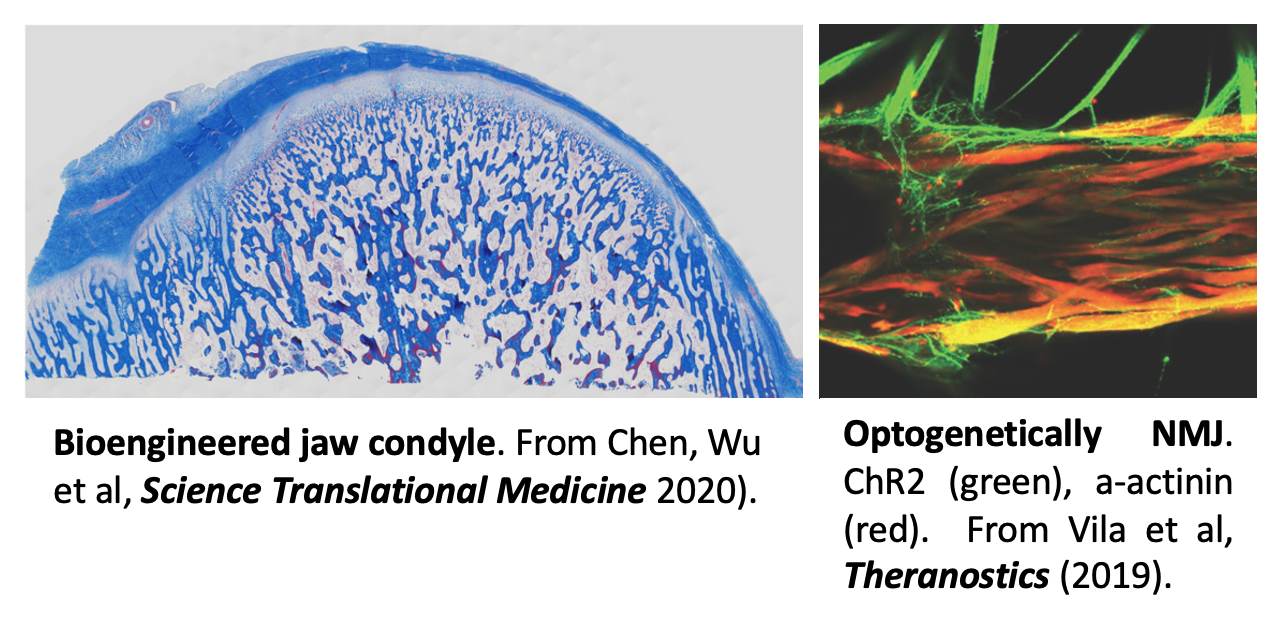
Defects in head and face due to trauma, tumor removal or congenital abnormalities not only leave patients with reduced tissue structure and function, but also render them psychologically scarred. The fundamental requirement for personalized bone reconstruction is to re-establish the functional and aesthetic properties of the original tissue by the living anatomically shaped grafts that integrate with the host tissues, connect to vascular supply, and support load-bearing and remodeling. Our approach involves the use of mesenchymal stem cells (from fat or bone marrow), anatomically shaped scaffolds (from mineralized bone matrix) and an advanced bioreactor with a corresponding anatomical chamber and dual flow, to bioengineer cartilage-bone grafts. In a recent study (Chen, Wu et al, Science Translational Medicine 2020), we engineered biologically matched grafts for repairing the lower jaw in a human-size swine model. Six months after implantation, the grafts maintained their anatomical structure and regenerated full-thickness, stratified, and mechanically robust cartilage over the underlying bone.
Neuromuscular junctions (NMJs) are the synapses between skeletal muscle fibers and motoneurons that are disrupted at early stages of various neuromuscular diseases, including Myasthenia gravis and Lou Gehrig’s disease. Human in vitro models of the NMJ provide a basis for both basic science insights and translational studies. We combined cell and tissue engineering with optogenetics, microfabrication, optoelectronics and video processing to generate light-responsive human NMJs (Vila et al, Theranostics 2019). We are using high-throughput platforms for automated diagnostics of NMJ disfunction using small samples of patients’ serum, and the measurements of effectiveness of new therapeutic modalities.
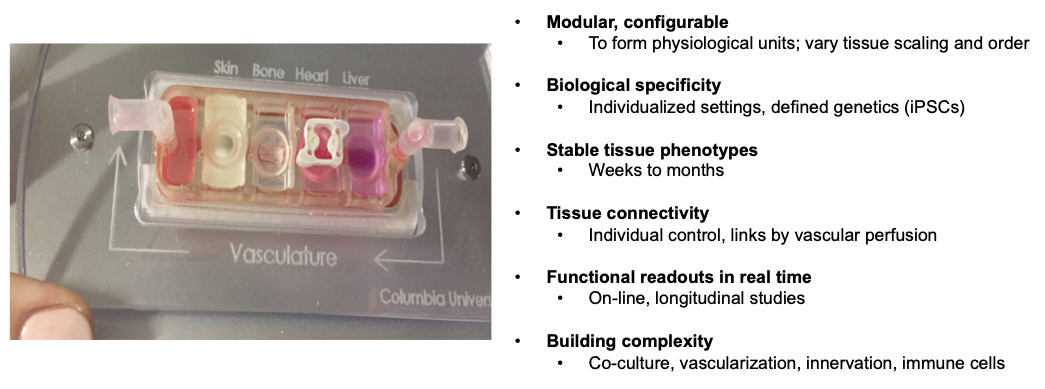
Cell culture and animal models often fail to recapitulate human physiology and the initiation and progression of diseases, with cancer as a notable example. Modeling integrated human physiology in vitro, using miniature human tissues connected into physiological units, has potential to transform biological research and healthcare. While very simple compared to their native counterparts, these tissues can recapitulate certain organ functions without the need to recapitulate the entire biological complexity. Our laboratory is developing a modular “organs on a chip” platform in which a set of human tissues of interest can be configured into a physiologically meaningful unit, and connected by vascular perfusion containing circulating cells (Ronaldson-Bouchard and Vunjak-Novakovic, Cell Stem Cell, 2018). All tissues can be formed from the same iPS cells, to allow individualized approaches to modeling organ functions in health and disease. Human tissues available for use in these platforms includes heart muscle, innervated skeletal muscle, bone, bone marrow, liver, vasculature, skin, solid tumors and immune components. We showed that the tissues can be matured and maintained for long periods of time, while allowing their communication by circulating cells, extracellular vesicles and molecular factors. We are using the “organs on a chip” platforms to investigate systemic pathologies, including off-target effects of drugs, inflammation, and cancer metastasis (to elucidate master regulators and predict drug sensitivity in metastatic cells).
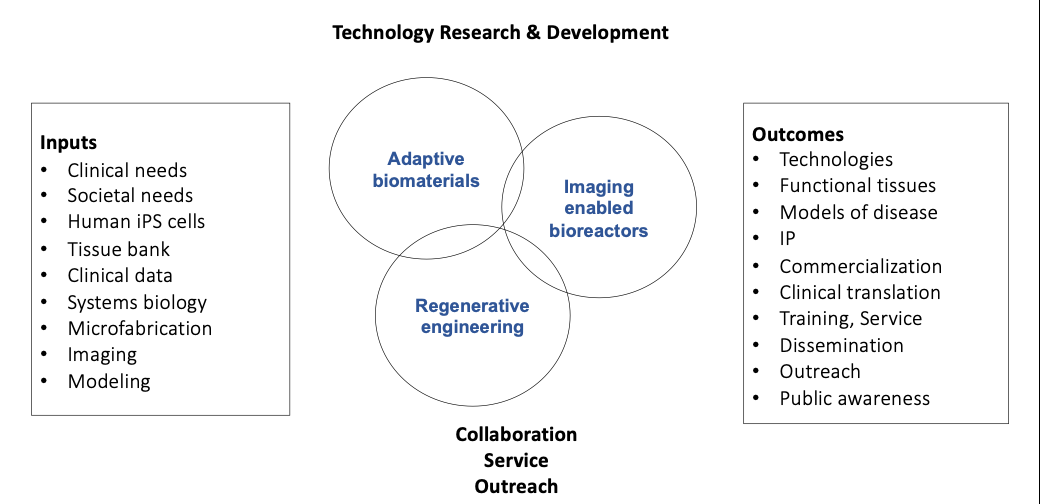
Tissue engineering is continuing to rapidly develop, but remains limited by the predetermined designs of scaffolds and bioreactors, and the lack of real-time, nondestructive measurements of cell and tissue function. To overcome these two limitations, we are investigating adaptive-responsive biomaterials that can sense environmental signals and actuate the cells, and imaging-enabled bioreactors that can provide real-time spatiotemporal control of cell and tissue function. The technical components of the NIH Tissue Engineering Resource Center (TERC) we are hosting are three Technology Research and Development Projects (Adaptive-responsive biomaterials; Imaging enabled bioreactor systems; Regenerative engineering), and a set of collaborative projects and service projects. We also pursue a robust and diverse training and dissemination program to support training of biomedical investigators in the use of new technologies, and the outreach to students, general public and underserved communities. Overall, our mission for the TERC is that it serves as a transformative driving force for the field of tissue engineering, by developing and translating new technologies, providing training and service, and offering dissemination and outreach programs.